Tips to Avoid 483 letters
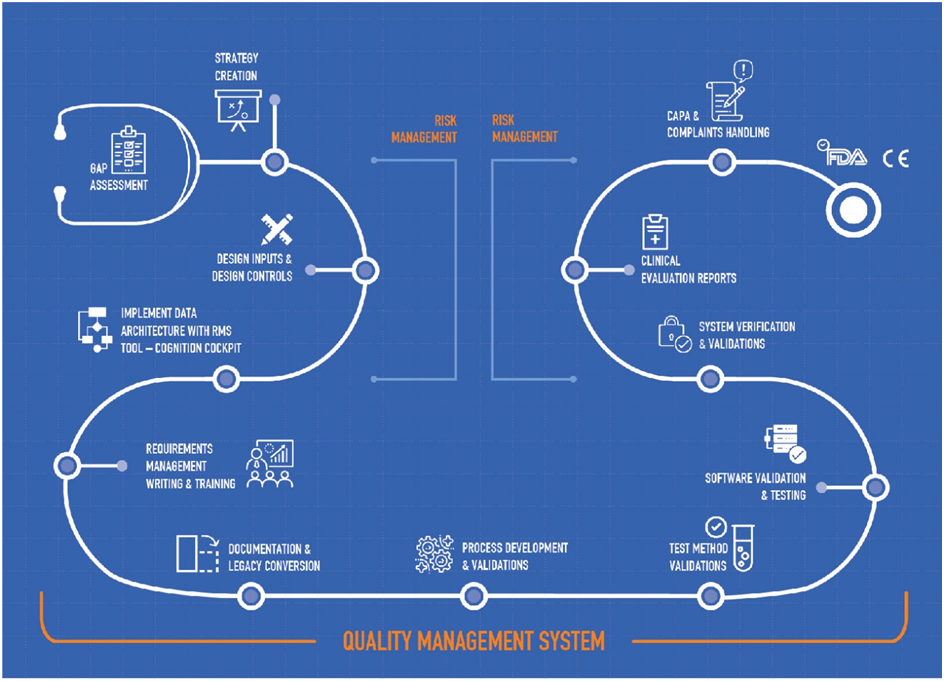
The U.S. Food and Drug Administration (FDA) is authorized to perform random inspections and audits, and these inspections can lead to FDA warning letters and FDA Form 483 observations. Medical device manufacturers receive observations ( Form 483 ) and / or warning letters on completion of USFDA Audit. The document outlines any violations of Good Manufacturing Practices (GMP’s) such as the facility, equipment, processes, controls, products, employee practices or records. Some tips to avoid a warning letter and form 483 observation – · Be Inspection Ready · Clearly Written Standard Operating Procedures · Proper Document Control · Develop a Compliance Culture · Correct Observations in Real-time · ...


